Showing all 4 results
-
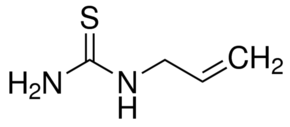
Allylthiourea
Read moreMolecular Formula C4H8N2S Synonyms ALLYLTHIOUREA
N-Allylthiourea
109-57-9
Thiosinamine
1-Allyl-2-thiourea
-
Ammonium Ceric Nitrate
Read moreIn organic synthesis, CAN is useful as an oxidant for many functional groups (alcohols, phenols, and ethers) as well as C–H bonds, especially those that are benzylic. Alkenes undergo dinitroxylation, although the outcome is solvent-dependent. Quinones are produced from catechols and hydroquinones and even nitroalkanes are oxidized. CAN provides an alternative to the Nef reaction; for example, for ketomacrolide synthesis where complicating side reactions usually encountered using other reagents. Oxidative halogenation can be promoted by CAN as an in situ oxidant for benzylic bromination, and the iodination of ketones and uracil derivatives.
-

Ammonium Reineckate Reineck salt
Read moreReinecke’s salt is a chemical compound with the formula NH4[Cr(NCS)4(NH3)2]·H2O. The dark-red crystalline compound is soluble in boiling water, acetone, and ethanol. The chromium atom is surrounded by six nitrogen atoms in an octahedral geometry. The NH3 ligands are mutually trans and the Cr–NCS groups are linear. The salt crystallizes with one molecule of water.
Properties Chemical formulaC4H12N7OCrS4 Molar mass 354.42 g/mol Appearance dark red solid Density 1.49 g/cm3 Melting point 270 °C (518 °F; 543 K) Boiling point decomposes Solubility in watersoluble in hot water -

Ceric Sulfate
Read moreCerium(IV) sulfate, also called ceric sulfate, is an inorganic compound. It exists as the anhydrous salt Ce(SO4)2 as well as a few hydrated forms: Ce(SO4)2(H2O)x, with x equal to 4, 8, or 12.
Uses:
The ceric ion is a strong oxidizer, especially under acidic conditions. If ceric sulfate is added to dilute hydrochloric acid, then elemental chlorine is formed, albeit slowly. With stronger reducing agents it reacts much faster. For example, with sulfite in acidic environments it reacts quickly and completely.
When ceric compounds are reduced, so-called cerous compounds are formed. The reaction taking place is:
- Ce4+ + e− → Ce3+
The cerous ion is colorless.
